EASY
JEE Main
IMPORTANT
Earn 100
Identify the graph(s) which correctly represents an isotherm at two temperatures and
(a)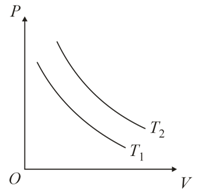

(b)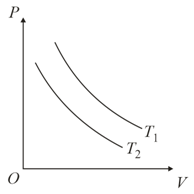

(c)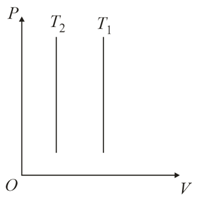

(d)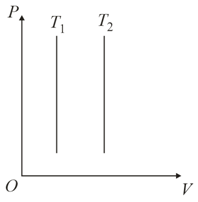

20% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
EASY
JEE Main
IMPORTANT
Two pistons can move freely inside a horizontal cylinder having two sections of unequal cross-sections. The pistons are joined by an inextensible, light string and some gas is enclosed between the pistons. On heating the system, the piston will
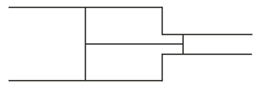
EASY
JEE Main
IMPORTANT
Two different masses of a gas and are heated separately in vessels of equal volume. The curve for mass makes angle an with -axis and that for mass takes angle an with -axis then
EASY
JEE Main
IMPORTANT
An ideal gas mixture filled inside a balloon expands according to the relation constant. The temperature inside the balloon is
EASY
JEE Main
IMPORTANT
A container of volume is divided into two equal compartments by a partition. One of these compartments contains an ideal gas at . The other compartment is vacuum. The whole system is thermally isolated from its surroundings. The partition is removed in the gas container. Its temperature now would be
EASY
JEE Main
IMPORTANT
One mole of helium is adiabatically expanded from its initial state to its final state . The decrease in the internal energy associated with this expansion is equal to
EASY
JEE Main
IMPORTANT
One mole of a perfect gas in a cylinder fitted with a piston has a pressure volume and temperature . If the temperature is increased by keeping pressure constant, then increase in volume is
EASY
JEE Main
IMPORTANT
Unit mass of a liquid with volume is completely changed into a gas of volume at a constant external pressure and temperature . If the latent heat of evaporation is , then the increase in the internal energy item is
EASY
JEE Main
IMPORTANT
A gas mixture consists of moles of oxygen and moles of argon at temperature . Neglecting all vibrational modes, the total internal energy of the system is
